Taurolidine: From early discovery to current practice
Exploring the namesake of TauroLock™
All of our lock solutions have one thing in common: The active ingredient taurolidine. In the past few decades, this substance has stirred up plenty of conversation. Scholars have suggestively deemed it a “new weapon” [1] and even “silver bullet” [2] in different medical contexts. What makes taurolidine so potent in the eyes of many experts? Where does it come from, how is it used today, and have we even unlocked its full potential yet? Let’s take a closer look at the science behind the ingredient that set the groundwork for TauroPharm and its products.
Overview
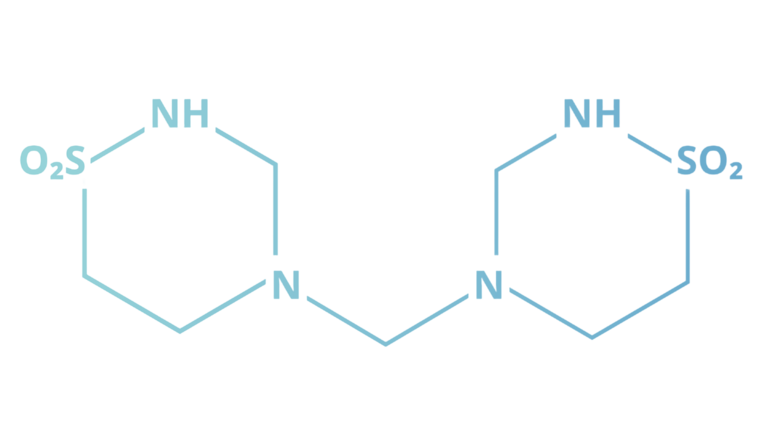
Speaking in chemical terms, taurolidine is a small dimeric molecule that is derived from the amino acid taurine. [3] Taurine naturally occurs in the human body and can also be found in many energy drinks. (According to a 2023 study, it might even have anti-aging properties!) [4]
In the 1970s, the Swiss company Geistlich-Pharma started producing taurolidine as a new chemical entity. In 1985, Browne et al. published a review that summarised research findings about the novel antimicrobial agent available thus far. Addressing the issue of surgical sepsis, the authors noted that “we must now look for a new weapon ... destroying all the germs” and taurolidine “may fill this need”. [1] Their assessment was based on previous in-vitro studies which had demonstrated “extremely sensitive” reactions of microorganisms to taurolidine, with “no evidence of systemic toxicity”. [1] Taurolidine had also been tested in clinical settings (“in vivo”) for the treatment of peritonitis, leading to “statistically significant” improvements without complications. [1]. It took another decade until these early findings were followed up by further studies. By the beginning of the 21st century, the discourse around taurolidine and its antimicrobial potency finally picked up speed.
Anti-tumour efficacy
In several earlier publications, taurolidine has been described as a “chemotherapeutic agent” [1,5] due to its impact on cancer cells. A 2000 study found that taurolidine exposure could inhibit the growth of tumour cells significantly, both in vitro and in vivo (when tested on animals). Further studies confirmed that it had the same effect on human tumour cells. [5,6]. The antineoplastic (i.e. anti-cancer) activity of taurolidine proved strong enough to “enhance apoptosis, inhibit angiogenesis, reduce tumour adherence, downregulate proinflammatory cytokine release, and stimulate anticancer immune regulation following surgical trauma”. [3]
Immunoregulation
The discovery of taurolidine’s anti-tumour efficacy went hand in hand with research on how it affects the immune system. While counteracting the proliferation of cancer cells, it also blocked the synthesis of IL-10 and TNF proteins. [7] These proteins act as inflammatory markers: When found at increased levels in the body, they indicate that the immune system is currently fighting off an infection. Lower levels of these proteins in turn indicate an improved inflammatory profile. [8] Scientists therefore came to the conclusion that taurolidine might be “an effective immunomodulatory agent in a clinical setting”. [9]

Antibiofilm efficacy
Findings on the anti-tumour and immunoregulative properties of taurolidine set the stage for another field of research: Its effect on so-called biofilm. Generally speaking, biofilm is a thin layer of microorganisms that grows on many wet surfaces. It provides a perfect breeding ground for germs and therefore poses a severe threat in clinical contexts. Oftentimes, the formation of biofilm is the first step in developing an infection. It not only invites microorganisms to accumulate and proliferate, but also makes them more resistant. Once they have settled, many microorganisms enter a state known as “dormancy”. They stop growing and become less sensitive to attacks from the immune system or antibiotic exposure. [10] Therefore, dormant or “mature” pathogens are particularly dangerous for patients at risk of infection. Taurolidine has proven itself as a useful agent to fight this threat. In the past 25 years, numerous studies have demonstrated how it not only prevents, but even eradicates biofilm that has already developed. [11,12,13,14]. Taurolidine exhibits a strong antimicrobial activity against a broad range of bacteria and fungi, especially those known to commonly cause infections. This includes vancomycin-resistant enterococci (VRE), methicillin-resistant S. aureus (MRSA), and Candida albicans, among others. [14] Scientists also found that taurolidine can inactivate more mature biofilm at stages where other antimicrobial agents become ineffective. [15]

As numerous studies had examined (and confirmed) the antimicrobial properties of taurolidine, the next question arose: Where and how might these properties be put to use? Towards the end of the 20th century, researchers increasingly began to explore possible applications in different medical fields. The primary objective has remained the same to this day: Effective treatment and, most importantly, prevention of infections.
Taurolidine to treat infection
Clinical evidence for taurolidine in the treatment of peritonitis (i.e. inflammation and infection within the abdominal cavity) was first published in the mid-1980s. Two randomised studies found its antimicrobial activity facilitated the healing process and reduced the risk of complications. [16,17,18] This was further substantiated by several publications during the 1990s. [18,19,20]
Most recently, a case report from Iran deemed taurolidine a “promising therapeutic option for challenging wound infections”. [21] The study group came to this conclusion after successfully treating a 17-year-old patient with a stage III ulcer. Antibiotics, regular debridement, and dressing changes were all in vain. But finally, irrigating the ulcer with a taurolidine solution resulted in progressive wound granulation and complete reepithelialisation after 2 months. The patient remained fully healed without any adverse effects after one year.
All in all, taurolidine has shown great potential as a therapeutic agent. Still, this potential has yet to be implemented and standardised in medical practice. Research on the treatment of peritonitis did, however, pave the way for another field of application: Lock solutions for central-venous catheters.
Taurolidine in lock solutions
Bacteria that cause peritonitis (f.ex., Pseudomonas aeruginosa or Staphylococcus aureus) [22] also commonly occur in biofilm in central-venous access devices (CVAD). It was therefore only a matter of time until scientists began exploring the potential of taurolidine as a lock solution.
CVADs (i.e. catheters and ports) are used in several contexts:
- In dialysis, they serve as the vessel through which the patient’s blood flows out of and back into the body.
- In oncology, they are implanted in patients receiving chemotherapy over the course of several months (or even years).
- In parenteral nutrition, they deliver nutrients intravenously for patients with chronic intestinal failure.
Central-venous catheters are lifelines in the most literal sense: Patients depend on them to survive. But they also carry the risk of complications that might lead to possibly life-threatening infections. Such complications often start with the formation of biofilm within the catheter: As bacteria and fungi accumulate and proliferate, they also grow more resistant over time. Once these pathogens enter the patient’s bloodstream, they can cause a catheter-related bloodstream infection (CRBSI) that might end deadly. Consequently, it is crucial to prevent biofilm formation in the first place. As illustrated above, clinical evidence has shown that taurolidine does exactly that. The founders of TauroPharmthus came up with the idea of taurolidine-based lock solutions for central-venous catheters.
Today, we have a range of products that all contain taurolidine as an antimicrobial agent. Four of these products also contain other active ingredients, to meet the individual needs of different patients: Tailor-made catheter lock solutions.
| Product | Ingredients | Recommended Fields of Application |
|---|---|---|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
Over the past 20 years, numerous studies have confirmed the efficacy of taurolidine-based lock solutions (and TauroLock™ products, specifically) in all relevant fields of application. The increasing amount of clinical evidence has in turn prompted national and international guidelines to include taurolidine in their recommendations. All of these publications (both studies and guidelines) can be accessed and downloaded on our website.
Taurolidine in cardiac surgery
Lately, taurolidine has garnered attention in another context: Prevention of CIED infection. CIEDs (short for cardiac implantable electronic devices, commonly known as pacemakers) come with a dilemma similar to that of CVADs: Patients depend on them to survive, but might also develop a potentially life-threatening infection from them. This risk has only increased in recent years for several reasons. On one hand, CIED procedures have become more complex (due to high-tech devices). On the other hand, the average patient has grown older and is therefore affected by more comorbidities. A review from 2024 [23] shed light on the alarming status quo, taking Germany as an example:
The study group analysed data provided by the country’s largest healthcare insurance fund from the year 2015. Out of 27+ million beneficiaries, they identified a cohort of more than 60,000 patients who underwent an invasive CIED procedure during that year. 2.6 % of all patients developed a CIED infection within 3 months after the procedure, with mortality rates of up to 15 %. Considering these alarming statistics, effective prevention of cardiac device infections is now more important than ever.
To solve this problem, TauroPharm has developed TauroPace™. Like TauroLock™ products, this solution also contains taurolidine as a further component with antimicrobial efficacy. When used in CIED procedures (placement, removal, or revision), it proved to protect patients against infections. As of 2025, two studies have confirmed the efficacy of TauroPace™.
- The first clinical trial was published in 2023. It reported that the use of TauroPace™; during 654 procedures on 631 patients resulted in 0.00 CIED infections (within the 3-month follow-up). [24]
- In 2024, an early report from the European registry shared the results of a clinical trial including 822 CIED procedures with the use of TauroPace™. The CIED infection rate amounted to 0.125 % three months post procedure. [25]
More studies are expected to be published in the foreseeable future, but the evidence available to date leaves little doubt about the potential of taurolidine in cardiac surgery. As Prof. Charles J. Love put it in an editorial commentary from 2023: “Taurolidine may be one step closer to the ‘Silver Bullet’ that will kill the microbes, prevent CIED infection, and not affect the host.” [2]
- Browne 1985. Pharmacological and clinical studies with Taurolin. 1985. Print.
- Love. EP Europace 2023. DOI: 10.1093/europace/euad332
- Neary et al. Ann Surg Oncol 2010. DOI: 10.1245/s10434-009-0867-9
- Singh et al. Science 2023. DOI: 10.1126/science.abn9257
- Shrayer et al. Anticancer Drugs 2003. DOI: 10.1097/00001813-200304000-00007
- Calabresi et al. Cancer Res 2001. PMID: 11559556
- Bedrosian et al. Cytokine 1991. DOI: 10.1016/1043-4666(91)90483-t
- Fontseré et al. Antimicrob Agents Chemother 2014. DOI: 10.1128/AAC.02421-14
- Härtel et al. J Microbiol Immunol Infect 2012. DOI: 10.1016/j.jmii.2012.04.00
- Rittershaus et al. Cell Host Microbe 2013. DOI:10.1016/j.chom.2013.05.012
- Torres-Viera et al. Antimicrob Agents Chemother 2000. DOI: 10.1128/AAC.44.6.1720-1724.2000
- Shah et al. Antimicrob Agents Chemother 2002. DOI: 10.1128/AAC.46.6.1674–1679.2002
- Pjatkowska et al. PLoS ONE 2021. DOI: 10.1371/journal.pone.0258148
- Visek et al. JPEN J Parenter Enteral Nutr 2025 DOI: 10.1002/jpen.2725
- Ocana et al. Abstract presented at ESPEN Congress 2024.
- Linder et al. 1980. Langenbecks Arch Chir Suppl Chir Forum 1980. Print.
- Aukland et al. in Brücker et al. Urban & Schwarzenberg 1985. Print.
- Reith. Langenbecks Arch Chir 1997. DOI: 10.1007/pl00014637
- Watson et al. J Leukoc Biol 1995. DOI: 10.1002/jlb.58.3.299
- Rosman et al. Eur Surg Res 1996. DOI: 10.1159/000129476
- Amanati et al. J Med Case Rep 2025. DOI: 10.1186/s13256-025-05094-5
- Barrios et al. J Vasc Access 2020. DOI: 10.1177/1129729820937099
- Baldauf et al. Scientific Reports 2024. DOI: 10.1038/s41598-024-82622-1
- Borov et al. EP Europace 2023. DOI: 10.1093/europace/euad306
- Vonthein et al. J Cardiothorac Surg 2024. DOI: 10.1186/s13019-024-03059-1


