Dialysis
A guide for patients
"Your kidneys no longer work properly." This sentence changes everything in a second. Many patients feel overwhelmed when their doctor first mentions the word "dialysis". Suddenly, it is no longer just a matter of tablets or diets, but a complex procedure necessary for years or even forever.
Dialysis goes beyond a therapy – it becomes a vital companion when the kidneys fail. This goes along with fundamental changes: regular, time-consuming sessions, side effects, and special requirements in everyday life. But the good news is: With appropriate preparation and support, dialysis allows you to lead an active, fulfilling life.
This guide speaks to both patients and their relatives. It explains how dialysis works, what challenges it poses, and how risks can be avoided. It aims to provide not only medical facts, but also practical tips and a dose of confidence. Because dialysis is not the end – it is merely a stepping stone on the journey with chronic kidney disease.
Overview
- Definition: What is dialysis?
- Who needs dialysis?
- The history of dialysis
- How does dialysis work?
- Types of dialysis explained
- Which access is used for dialysis?
- Everyday life with dialysis
- What do dialysis patients have to consider?
- Nutrition with dialysis
- Risks of dialysis
- Prevention in dialysis
- Medication and additional treatment
- Kidney transplants
- Dialysis soon to be obsolete?
- References
Definition: What is dialysis?

Kidneys are truly high performers: Every day, around 1,500 litres of blood flow through them, from which they form around 170 to 180 litres of primary urine and eventually 1.5 litres of final urine. In doing so, they filter toxins, regulate water and salt balance, control blood pressure, and produce hormones for blood formation and bone metabolism.
If these functions fail partially or completely, the condition is referred to as renal insufficiency. In early stages, deficits can be compensated for with medication, dietary changes and blood pressure control. When these remedies no longer work, dialysis comes into play. This is a replacement procedure that takes over the kidneys’ most essential tasks:
- removal of toxins from the blood
- regulation of fluid balance
- correcting salt and electrolyte imbalances
However, this method cannot fully replace the kidneys’ hormonal functions. Therefore, those affected also need medication that stimulates blood formation (erythropoietin) or regulates bone metabolism (vitamin D), for example.
People become dependent on dialysis for a variety of reasons. In general, we can distinguish between chronic and acute diagnoses.
End-stage chronic renal failure
In this case, the glomerular filtration rate (GFR) falls below 15 ml/min/1.73 m². As a result, the body is no longer able to adequately excrete metabolic waste products such as urea, uric acid, and creatinine. Common triggers are diabetes mellitus (diabetic nephropathy), high blood pressure (hypertensive nephropathy), chronic glomerulonephritis, or polycystic kidney disease.
Acute renal failure
Several circumstances can lead to short-term kidney failure. These include shock, sepsis, acute glomerulonephritis, toxic substances (e.g. medication, contrast agents), kidney stones, or urinary flow disorders. In these cases, dialysis is used as a temporary measure until the organs have recovered.
The first signs of renal insufficiency might easily be overlooked or attributed to other diseases. Many patients do not notice anything for a long time, as kidneys can still compensate even when their function is significantly impaired.
Early warning signs of renal insufficiency
- Changes in urine: more frequent or less frequent urination, foamy urine (indicating protein excretion), dark or reddish urine (indicating blood in the urine)
- Water retention (oedema): swollen feet, ankles, eyelids.
- Decreased performance & fatigue: Result of incipient anaemia.
- High blood pressure: Often the first objective sign, sometimes difficult to control.
Advanced symptoms of renal insufficiency
The more the organ function declines, the more substances which are normally excreted in urine accumulate in the blood. This contamination with urinary substances is called uraemia and can manifest itself in different symptoms:
- nausea, vomiting, loss of appetite
- itching, dry skin
- headaches, difficulty concentrating, confusion
- muscle cramps, restless legs syndrome
- shortness of breath (due to fluid overload or acidosis)
- cardiac arrhythmia (mainly due to increased potassium levels)
Whether the symptoms mentioned are actually caused by kidney failure can ultimately only be determined through comprehensive examinations. A specialist (nephrologist) will then make a diagnosis and prescribe dialysis treatment, if necessary.
The idea of filtering blood through a membrane originated in the 19th century. But it took several decades before a fully developed technique was used on humans.
- In 1854, Scottish chemist Thomas Graham first described the principle of dialysis: Certain molecules can diffuse through a membrane, while others cannot. Graham only used this knowledge for laboratory experiments.
- In 1945, Dutch physician Willem Kolff performed the first successful haemodialysis on a patient. His so-called "artificial kidney" consisted of a drum filter, which is more reminiscent of a washing machine today.
- In the 1960s, devices became more compact and reliable. From then on, dialysis patients could be treated for years on end.
Peritoneal dialysis was introduced in the 1970s. Thanks to this method, some patients could now receive treatment at home.
Today, dialysis has evolved into an established, safe procedure that accompanies millions of people worldwide in their everyday lives. Modern devices monitor blood pressure, fluid balance, and blood values in real time to adjust the treatment individually. The development of dialysis was a milestone in modern medicine – comparable to the invention of insulin for diabetics. It not only saves lives, but also enables a high quality of life.
Dialysis is based on a simple principle: Blood passes through a semi-permeable filter membrane that removes toxins and excess fluid, while important blood cells and proteins remain in the blood. In practice, this involves a complex interplay of various mechanisms.
- Diffusion: Small molecules such as urea or creatinine migrate from a highly concentrated fluid (blood) to a low concentrated fluid (dialysate). This removes waste products.
- Ultrafiltration: Pressure differences serve to "press" excess water out of the blood. This is crucial for patients prone to water retention, which would otherwise lead to oedema or heart failure.
- Osmosis: In peritoneal dialysis, the glucose in dialysate is used to draw fluid from the body into the dialysate.
How long does dialysis take?
A typical dialysis session lasts four to five hours and takes place three times a week. This never reaches the performance of healthy kidneys, which work around the clock. But it suffices to ensure survival – as long as patients strictly adhere to their treatment plan. Shortened or skipped sessions can be life-threatening.
Haemodialysis step by step
- Blood is fed through a vascular access into a so-called dialyzer (artificial kidney).
- The dialyzer contains fine hollow plastic fibres with walls that serve as a filter membrane.
- Blood flows on one side and a special dialysis fluid flows on the other.
- An exchange takes place between both sides: Toxins and excess salts migrate into the dialysate, while purified blood flows back into the body.
There are several procedures that differ in terms of technique, location, and impact on everyday life. We can mainly distinguish between haemodialysis (extracorporeal) and peritoneal dialysis (intracorporeal).
Haemodialysis (HD)
- Location: Dialysis centre or (less commonly) at home.
- Procedure: Blood is cleaned through an artificial kidney via a shunt or catheter.
- Duration: Approx. 4–5 hours, usually 3 sessions per week.
- Advantages: Very effective detoxification, close medical supervision.
- Disadvantages: Time-consuming, limited flexibility, possibly physical exhaustion after a session.
Peritoneal dialysis (PD)
- Location: Usually at home.
- Procedure: The body's own peritoneal membrane serves as a filter. Dialysis solution is poured into the abdominal cavity via a catheter. This solution absorbs toxins and fluid over several hours. The fluid is then drained again later.
- Variants:
CAPD (continuous ambulatory peritoneal dialysis): The bag is changed manually several times a day.
APD (automated peritoneal dialysis): A machine performs the exchange at night while the patient sleeps. - Advantages: Independence from the centre, flexible daily routine, often better blood pressure control.
- Disadvantages: Risk of peritonitis, daily personal responsibility, permanent abdominal catheter required.
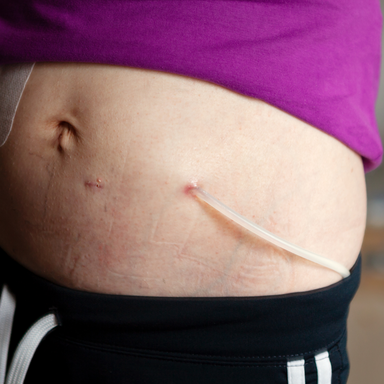
For peritoneal dialysis, patients need a special catheter (usually a Tenckhoff catheter). It is inserted into the abdominal cavity surgically or laparoscopically and exits through the skin on the side of the lower abdomen.
Haemodialysis, on the other hand, requires venous access. This should:
- ensure high blood flow (approx. 300–500 ml/min),
- be easily accessible for nursing staff,
- cause as little disruption as possible to patients' everyday lives.
There are various options available to meet these criteria. Which of these proves most suitable depends on individual factors.
Arteriovenous fistula
The AV fistula has become a standard in dialysis. A surgical procedure establishes a direct connection between an artery and a vein (usually in the forearm between the radial artery and the cephalic vein). The arterial blood flow causes the vein to expand and its wall to thicken so that it becomes a "shunt vein" to be punctured regularly.
Advantages:
- Longevity over many years
- Lowest risk of infection
- High blood flow
Disadvantages:
- Several weeks to months of maturation time
- Surgical intervention necessary
- Risk of complications such as stenosis, thrombosis, or aneurysms
Arteriovenous graft (vascular prosthesis)
If a patient has no suitable vein, a vascular prosthesis can be implanted between the artery and vein. The graft is then punctured like a vein.
Advantages:
- Ready for use after 2-3 weeks
- Also possible in cases of poor vein conditions
Disadvantages:
- Higher rate of thrombosis and infection compared to AV fistulas
- Lower durability (often only a few years)
Central-venous catheters
If a fistula or shunt is not an option, patients have a third alternative: A central-venous catheter (CVC) provides access through a vein close to the heart. We can differentiate two types of CVC:
- Non-tunnelled CVCs are used for short-term treatment, e.g. in acute renal failure or when a shunt still needs to mature. They can be inserted without surgery, but can only remain for a few days to weeks.
- Tunnelled CVCs are "tunnelled" under the skin and have a fixation cuff (Dacron cuff). This allows them to grow into the tissue. Tunnelled CVCs have a lower risk of infection than non-tunnelled ones and can remain in the body for months or even years.
A glimpse inside a modern dialysis centre in Brazil. Source: Nefrostar
A glimpse inside a modern dialysis centre in Brazil. Source: Nefrostar
A glimpse inside a modern dialysis centre in Brazil. Source: Nefrostar
A glimpse inside a modern dialysis centre in Brazil. Source: Nefrostar
A glimpse inside a modern dialysis centre in Brazil. Source: Nefrostar
Dialysis is not an easy therapy that can be done "on the side". It shapes everyday life in many ways – practically, physically, mentally. However, this does not mean that “normal” life, as it was before, comes to a halt. Dialysis entails unusual challenges – but these challenges can be mastered with good planning and support from specialists and relatives.
Time commitment
- Haemodialysis: Usually 3 sessions per week lasting 4–5 hours each, plus travel time, waiting period, and recovery after the session. For many, this adds up to 20–25 hours per week.
- Peritoneal dialysis: Regular bag changes (CAPD) or night-time therapy with a device (APD). Often more flexible in terms of time, but requiring a consistent routine.
Physical effects
Many patients report fatigue, dizziness, or headaches post treatment. Some also feel relieved afterwards because excess fluid has been removed.
Peritoneal dialysis is often more continuous and consistent. However, patients can feel the catheter in their abdomen and have to live with a permanent "bloated feeling".
Personal implications
Many people can still work while on dialysis – sometimes part-time, sometimes with flexible hours. Legally, dialysis times are usually protected as "sick leave". Family members have to adjust to new daily routines. Especially in cases of home dialysis, they often take on important tasks.
Sports, travelling, and hobbies are still possible – but involve more effort. If dialysis patients want to go on holiday, for example, they must make sure that they can visit a dialysis centre at their destination.
Last but not least, the emotional strain caused by dialysis should not be underestimated. Those affected must learn to deal with a sense of dependence, loss of control, or anxiety. They can find support in various ways: Apart from professional counselling, talking to other people in the same situation (e.g. through self-help groups or social media) can prove very helpful.
Dialysis comes with great responsibility. Those affected face numerous risks – but most complications can be avoided with consistent treatment plans. Most patients have a fixed schedule for checking blood values, ECG, heart ultrasound and bone density measurements. All of this serves to detect (and treat, if necessary) side effects and long-term consequences of renal insufficiency at an early stage.
The basics of everyday dialysis
- Regular sessions – do not miss any appointments, even if you feel "well".
- Taking medication – e.g. blood pressure medication, erythropoietin, phosphate binders.
- Stable fluid balance – drinking too much can be life-threatening, since the kidneys cannot excrete the fluid.
- Weight control – daily check-ins help to detect fluid retention early on.
- Hygiene at the access site – handle the shunt or catheter with utmost care to prevent infections.
- Monitoring of blood values – potassium, phosphate, and haemoglobin are particularly important.

Tips for haemodialysis patients |
Tips for peritoneal dialysis patients |
|
|
You are what you eat: This holds particularly true for dialysis patients, as they can no longer completely excrete the minerals absorbed through food. No foods are strictly "forbidden" – rather, it is the dose that makes the poison. Those suffering from kidney failure must therefore pay careful attention to what and how much they eat and drink.
- Excessive fluid intake must be avoided at all costs, since this can lead to high blood pressure, oedema, or heart problems. The recommendation for daily fluid intake ranges around 500 ml, plus the amount of urine excreted. Ice cubes, sour sweets, or chewing gum can help if you feel thirsty.
- Extremely high potassium levels can cause cardiac arrhythmia. Dialysis patients should avoid foods containing potassium (e.g. bananas, oranges, potatoes, tomatoes, nuts, and dried fruit). The potassium content in vegetables can also be reduced by soaking or boiling twice.
- Too much phosphate damages bones and blood vessels. Soft drinks such as Coca Cola, processed cheese, nuts, and wholegrain products in large quantities are not recommended. Phosphate binders (in tablet form) can be used to neutralise the phosphate intake.
- Dialysis increases the need for protein because the body loses a certain amount during each treatment. Patients should therefore ensure they eat a protein-rich diet (lean meat, fish, eggs, dairy products, or vegan alternatives).
- Salt binds water and should be used sparingly. Highly processed products such as ready-made meals, crisps, and sausages are not a good choice for people with kidney failure.

Potassium levels in different foods
| High potassium (>250 mg / 100 g) | Low potassium (<150 mg / 100 g) |
| Bananas Oranges, orange juice Kiwis Dried fruit (raisins, dates, apricots) Avocado Potatoes, sweet potatoes Tomatoes, tomato juice Spinach, Swiss chard, kale Broccoli, Brussels sprouts Legumes (lentils, beans, chickpeas) Nuts, almonds, hazelnuts Chocolate, cocoa Meat (especially beef and pork) Fish (e.g. salmon, tuna) |
Apples Pears Blueberries Strawberries Grapes Cucumbers Courgettes Lettuce Chinese cabbage White bread, toast Rice, pasta (cooked) Soft cheese (e.g. cream cheese, quark) Eggs Chicken (small portions) |
In addition, dialysis patients should also incorporate light to moderate exercise into their daily routine. Activities such as cycling, swimming, walking, or strength training improve energy levels and quality of life in the long term. Under certain conditions, it may even be possible to run a marathon!
Dialysis is a life-saving therapy. Nevertheless, it can come with unpleasant or even dangerous side effects. Some occur acutely during individual sessions, others develop slowly over time.
Acute complications during haemodialysis
- A drop in blood pressure (hypotension) is the most common side effect and triggered by rapid fluid removal. It manifests itself through symptoms such as dizziness, nausea, or weakness.
- Muscle cramps often occur at the end of dialysis due to electrolyte shifts.
- Nausea or headaches can occur due to fluctuations in blood pressure or an increase in metabolic products.
- Allergic reactions are rare but can be caused by dialysis filters or medication.
- Post-treatment bleeding is possible if the blood needs to be “thinned” with heparin.
Long-term complications of dialysis
- Cardiovascular diseases pose a high risk for dialysis patients. Causes can include chronic high blood pressure, vascular calcification, or overhydration.
- Bone and mineral disorders can develop as a result of changes in calcium and phosphate levels. In these cases, the condition is referred to as renal (i.e. kidney-related) osteopathy.
- Anaemia can develop over time because the kidneys no longer produce erythropoietin.
- Amyloidosis (accumulation of proteins in joints and tendons) can be a side effect after many years of dialysis.
- Infections can spread if bacteria enter the venous access site (and from there into the bloodstream).
Catheter-related complications in dialysis
Most dialysis patients have a shunt as vascular access. Some need a central-venous catheter – either temporarily (e.g. while the shunt matures) or permanently if the vessels are not suitable for a shunt. CVCs offer the advantage of being ready for immediate use. At the same time, however, they carry particular risks:
- Infections are considered the most common and dangerous complication. They can occur locally at the exit site or spread into the blood through the catheter. The latter is called a catheter-related bloodstream infection (CRBSI). In the worst case, this can lead to sepsis. Sepsis can be fatal and must therefore be treated immediately.
- Occlusions (blockages in the catheter) cause problems for many dialysis patients. This results in a reduced flow rate: Blood cannot flow through the catheter quickly enough during the cleaning process.
- Thrombosis occurs when a blood clot (thrombus) forms and narrows or even completely blocks the vessel. This can have serious consequences, including pulmonary embolism or strokes.
- Mechanical problems can also occur, e.g. if the catheter slips (dislocation), kinks, or breaks.
When complications arise, they often result in long hospital stays and surgical procedures. At worst, patients pay with their lives. Preventive measures are therefore essential in dialysis. This includes a healthy diet and adherence to highest hygiene standards. Catheter care plays a very important role in this regard. Patients should closely monitor their own condition and report any unusual symptoms as soon as possible. Chills, fever, or inflammation at the exit site, for example, might indicate a bloodstream infection.
Recommendations for haemodialysis
- Slow fluid removal – for patients prone to drops in blood pressure.
- Appropriate dialysis dose – individually tailored, measured e.g. by Kt/V value.
- Shunt care – gentle handling of the arm, no blood pressure measurement or blood sampling, daily palpation for flow noise.
- Infection prevention – strict hygiene during puncture.
Recommendations for peritoneal dialysis
- Hygiene when changing bags: Wash hands thoroughly, wear a mask, keep the catheter site clean.
- Catheter care: Daily inspection, prompt reporting of redness or pain.
- Training: Patients and relatives usually receive special training before performing dialysis independently at home.
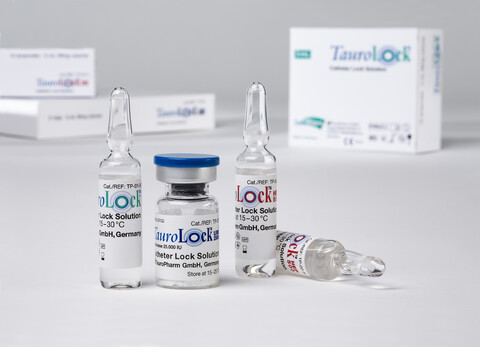
Lock solutions in dialysis
To avoid catheter-related complications, the exit site must be kept completely sterile. In addition, the catheter should be flushed with a physiological saline solution after each session. Experts recommend the pulsatile flushing technique with a 2x10 ml saline solution syringe. [1]
However, these measures alone are not sufficient. For optimal protection, patients need an antimicrobial lock solution. This solution serves to securely close or block the catheter so that no germs can accumulate. Ideally, a catheter should be blocked regularly after each dialysis session. The lock solution then remains in the lumen until the next session. This method significantly reduces the risk of biofilm (i.e. the colonisation of the catheter with pathogens).
Lock solutions developed by TauroPharm contain taurolidine. This active ingredient is capable of killing a wide range of bacteria and fungi without causing resistance. Taurolidine therefore ensures a strong prophylaxis against infection. To prevent other complications, TauroLock™ solutions contain additional active ingredients.
- Citrate (4 %) and/or heparin (in various concentrations) to prevent occlusion
- Urokinase to prevent occlusion and thrombosis
| Product | Ingredients | Recommended Fields of Application |
|---|---|---|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
For dialysis patients prone to flow problems, we recommend the 2+1 protocol: Locking twice a week with TauroLock™-HEP500 and once a week with TauroLock™-U25.000. This has proven highly effective in several clinical studies. [2,3]
Numerous guidelines now recommend taurolidine-based lock solutions in dialysis, including those of the German Society of Nephrology. [4,5] The Dutch Association of Medical Specialists, for example, also recommends blocking with a thrombolytic agent once a week, in accordance with the 2+1 protocol. [6]
Dialysis procedures can never fully replace the kidneys’ function. Therefore, those affected must resort to other means to ensure the best possible care. These include (as needed):
- ACE inhibitors, AT1 blockers, beta blockers to lower blood pressure and protect the heart and blood vessels
- erythropoietin (EPO) and iron supplements to treat anaemia
- phosphate binders, vitamin D analogues, and calcium supplements for mineral metabolism
- heparin to prevent blood clotting during haemodialysis
- diuretics (water tablets) to stimulate kidney activity (if residual function is present)
- statins to lower blood lipid levels
- antibiotics (to treat infections)
For many patients, dialysis only serves as a temporary solution. The best treatment for end-stage renal failure is an organ donation. There are two options:
- Living donation (commonly from relatives or close friends)
- Post-mortem donation (usually involving a waiting period of several years)
If the procedure turns out successful and the body accepts the new kidney, it can remain functional for decades. The average lifespan of a transplanted kidney ranges between 10 and 15 years. Thus, transplant allow for a significantly better quality of life than dialysis and are considered the medically favourable option in the long run.
Requirements for a kidney transplant
- physiological examinations (heart, lungs, infections, tumour exclusion)
- no serious contraindications (e.g. active cancer, severe heart failure)
- mental stability
- willingness to take medication for life
Risks of a kidney transplant
- Rejection reaction if the immune system classifies the kidney as "foreign".
- Increased risk of infections or cancer, facilitated by lifelong immunosuppression.
- Side effects of medication, e.g. high blood pressure, diabetes, weight gain.
In general, even a donated kidney does not guarantee a carefree life – yet for many patients, it offers a key to more freedom. If a transplant is not possible or fails, dialysis remains indispensable.


Optimising dialysis
- Nanotechnology is used to develop filters that can capture toxins more precisely.
- Biocompatible membranes reduce the risk of immune reactions and inflammation.
- Telemedicine enables home dialysis patients to transmit their data directly to the responsible centre.
- In years to come, artificial intelligence could help to control dialysis parameters individually and dynamically.
Alternatives to conventional dialysis
- Portable dialysis devices can be carried like a small backpack. This would mean that patients would no longer depend on dialysis centres. The first models have already been tested.
- Wearable artificial kidneys (WAK) are portable devices designed to filter continuously (instead of three times a week). This would give patients more flexibility in their everyday lives.
- Bioartificial kidneys are based on a combination of technology and living cells. They are intended to take over not only the cleansing function of the kidney, but also its hormonal functions.
- Goossens. Dialysis & Apharesis 2015.
- Al-Ali et al. Nephrol Dial Transplant 2018. DOI: 10.1093/ndt/gfx187
- Winnicki et al. Kidney Int 2018. DOI: 10.1016/j.kint.2017.06.026
- German Society of Nephrology (DGfN) 2019. Print.
- German Society of Nephrology (DGfN) 2022. Print.
- Dutch Federation of Medical Specialists (Federatie van Medisch Specialisten) 2022. Print.



